Covid Vaccine Pregnancy Trial
The pandemic has shone a light on the historic failing and exclusion of. Pregnant women are at increased risk of morbidity and mortality from COVID-19 but have been excluded from the phase 3 COVID-19 vaccine trials.

Is The Covid Vaccine Safe If Your Pregnant Cleveland Clinic
In addition to vaccination protecting women against Covid-19 and its complications during pregnancy emerging evidence has shown transplacental transfer of severe acute respiratory syndrome.

Covid vaccine pregnancy trial. Some COVID-19 vaccine makers have since planned to enroll pregnant people in their clinical trials. A COVID-19 vaccine from 30 days prior to the first day of the LMP to end of pregnancy or a negative screening test for SARS-CoV-2 during pregnancy Living in the countries where at least one COVID-19 vaccine is marketed. From December 14 2020 to February 28 2021 we used data from the v-safe after vaccination health checker surveillance system the v-safe pregnancy registry and the Vaccine Adverse Event Reporting System VAERS to characterize the initial safety of mRNA Covid-19 vaccines in pregnant persons.
To evaluate the immunogenicity of COVID-19 messenger RNA mRNA vaccines in pregnant and lactating. Despite ACOGs persistent advocacy for the inclusion of pregnant individuals in COVID-19 vaccine trials none of the COVID-19 vaccines approved under EUA have been tested in pregnant individuals. Vaccines that use the same viral vector have been given to pregnant people in all trimesters of pregnancy including in a large-scale Ebola vaccination trial.
Clinical trials have shown that COVID-19 vaccines are remarkably effective in protecting those age 12 and up against infection by the coronavirus SARS-CoV-2. The Joint Committee on Vaccination and Immunisation JCVI has advised that pregnant women should be offered COVID-19 vaccines at the same time as people of the same age or risk group. The truth about pregnancy risks and COVID-19 vaccines - Los.
The expectation was that they would work just as well to protect pregnant women. However studies in pregnant women have begun and post-market surveillance is underway. A clinical trial called Preg-CoV has been launched to help determine the best gap between doses for pregnant women as well as exploring in greater detail potential side-effects and the impact on.
The Moderna COVID-19 Vaccine Pregnancy Registry will collect primary data from pregnant women who have received the Moderna COVID-19 vaccine and their healthcare providers HCPs. Pregnant women are more likely to develop severe COVID-19 or die from the disease but are excluded from clinical trials with new vaccines. Two doses of the PfizerBioNTech COVID-19 vaccine were safe and 78 effective in preventing infection in pregnant women in a real-world study in Israel.
We have summarized several academic papers that investigate outcomes of COVID-19 infection and the vaccines against it among pregnant individuals to help journalists bolster their reporting with data. The placebo-controlled observer-blinded study will track the safety tolerability and immunogenicity of two doses of vaccine 21 days apart compared to placebo for 7 to 10 months. By Kezia Parkins 22 Jul 2021 Last Updated August 11th 2021 1520 Studies testing the safety of Covid jabs in pregnant people have only just begun leaving some hesitant to undergo vaccination.
Although there is not yet pregnancy-specific data about COVID vaccines from clinical trials the vaccines have been studied in pregnant laboratory animals. No adverse pregnancy-related outcomes including adverse. Studies Confirm COVID-19 mRNA Vaccines Safe Effective for Pregnant Women.
This means there is currently very limited clinical trial data on the immune response and side effects caused by. The countrys largest clinical trial investigating the best gap between first and second COVID-19 vaccine doses for pregnant women is being launched in England today Tuesday 3 August. A new observational study has begun to evaluate the immune responses generated by COVID-19 vaccines administered to pregnant or postpartum people.
Researchers will measure the development and durability of antibodies against SARS-CoV-2 the virus that causes COVID-19 in people vaccinated during pregnancy or the first two postpartum months. Up to 1 year. The initial advice from immunisation expert groups was therefore cautious and COVID-19 vaccines were not routinely recommended in pregnancy.
Pfizer and BioNTech say the first US. It is notable that as of April 26 2021 more than 100000 pregnant women reported having received a Covid-19 vaccination and yet only a small fraction 47 have enrolled in the v-safe pregnancy. Participants have been given shots in a large-scale clinical trial to assess the safety and efficacy of.
Number of Participants Having Infants With Suspected Major and Minor Congenital Malformations Time Frame. No adverse pregnancy-related outcomes occurred in previous clinical trials that used the same vaccine platform as the JJJanssen COVID-19 vaccine. Pfizer is launching a clinical trial testing approximately 4000 pregnant women with the companys COVID-19 vaccine the company announced Thursday.
Lack of trials in pregnant people driving Covid-19 vaccine hesitancy. Pregnant people were not included in the first clinical trials for COVID-19 vaccines so at the time of initial guidance there was limited evidence confirming the safety of COVID-19 vaccines during pregnancy. The exclusion of pregnant and lactating women from COVID-19 vaccine trials Text Box 1 reflects a historic pattern of protection by exclusion representing an instance in which the estimated effect of a therapy on mother and child will rely on anecdotal and delayed reports from healthcare settings rather than the monitored setting of a clinical trial2 This exclusion is not.
Last week February 18 Pfizer and BioNTech announced a global Phase 23 trial of their COVID-19 vaccine in 4000 healthy pregnant women. A long-standing tradition of keeping pregnant women out of clinical trials is having serious consequences with regard to COVID-19. Called developmental and reproductive toxicity DART studies research with pregnant animals can provide reassurance about moving forward with vaccine research in pregnant people.
Led by researchers at Maccabi Healthcare Services in Tel Aviv the retrospective observational study was published yesterday in JAMA. Data on vaccine safety and immunogenicity in these populations are therefore limited.

Women S Views On Covid 19 Vaccination During Pregnancy A Uk Based Study

Should Pregnant Women Get Covid 19 Vaccine Youtube

Should I Get The Covid 19 Vaccine While Pregnant Or Breastfeeding Experts Explain The Safety Evidence And Clinical Trials
Why Covid Vaccines Are Likely Safe For Pregnant People Scientific American
Covid 19 Vaccines And Pregnancy Johns Hopkins Bloomberg School Of Public Health
Pfizer Study Of Covid 19 Vaccine In Pregnant Women Delayed By Slow Enrollment Wsj

Should Pregnant Women Be Vaccinated Against Covid 19

Coronavirus Disease 2019 Vaccine Response In Pregnant And Lactating Women A Cohort Study American Journal Of Obstetrics Gynecology
Pfizer And Moderna Are Safe And Effective In Pregnant Women Provide Antibodies To Newborns Abc News
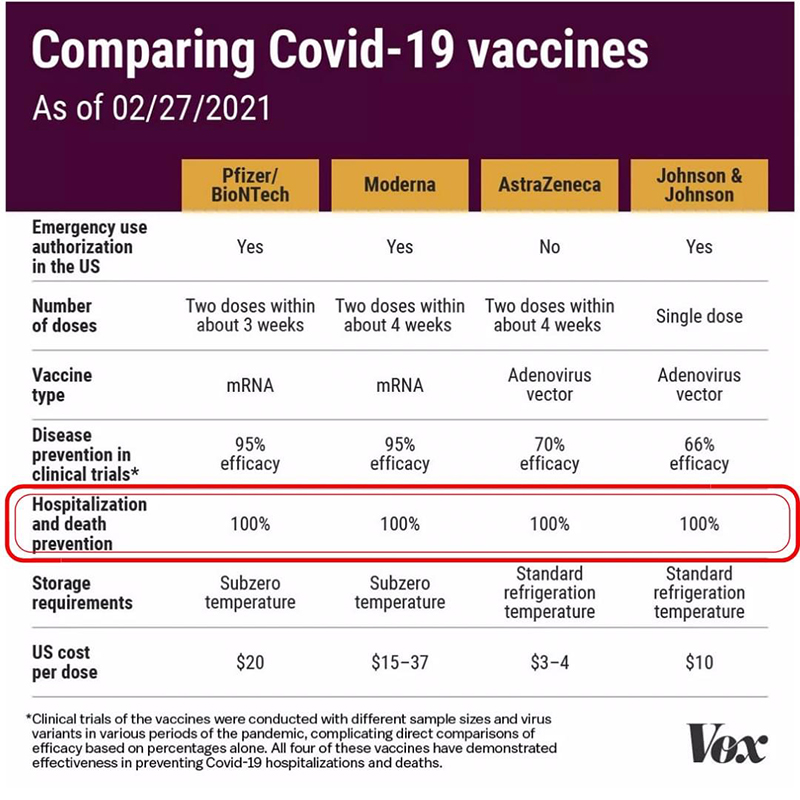
Covid 19 In Pregnancy Maternal Care Maternal Fetal Care High Risk Obstetrics Ur Medicine Obstetrics Gynecology University Of Rochester Medical Center

Covid 19 Vaccination Considerations For Pregnant People Patient Care
No Placental Damage Observed In Case Study Of Pregnant Women With Mrna Covid 19 Vaccinations

U S Begins Clinical Trial Testing Covid 19 Vaccine In Pregnant Women Ctv News
Nih Begins Clinical Trial Testing Covid 19 Vaccine In Pregnant Women Reuters
More Risks To Pregnant Women Their Newborns From Covid 19 Than Known Before Study Reuters

Cdc Recommends Pregnant Women Get Covid Vaccine After Study Shows It S Safe
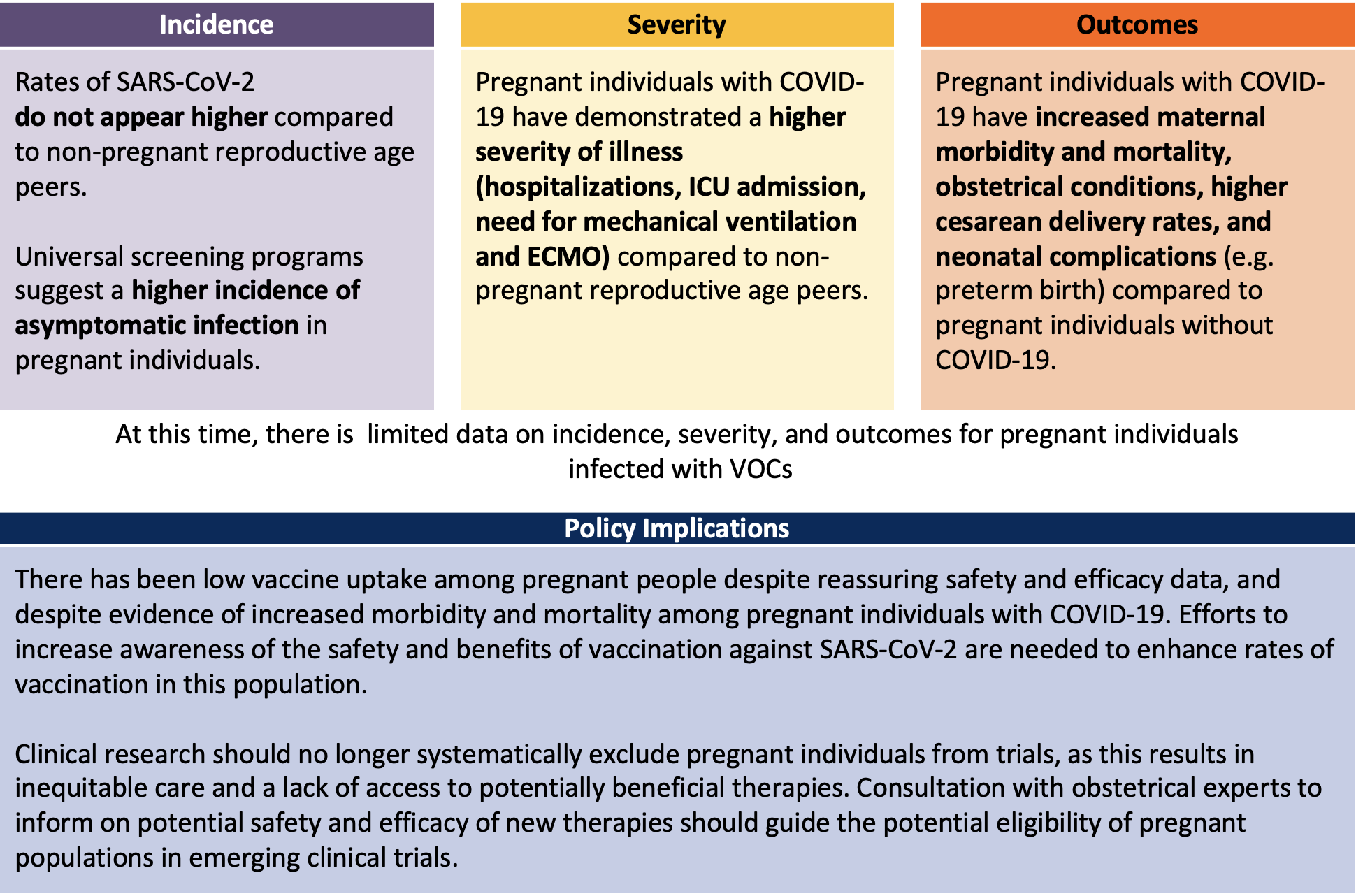
The Incidence Severity And Management Of Covid 19 In Critically Ill Pregnant Individuals Ontario Covid 19 Science Advisory Table

Covid Vaccines Protect Pregnant Women Study Confirms The New York Times

Q A New Trials Tackle Covid 19 Vaccines In Pregnancy The Scientist Magazine
